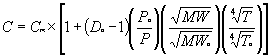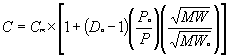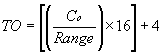|
|||
|
Authors: Russell S. Berry Charles E. Dene Abstract Over the last five years continuous emissions monitoring system (CEMS) operators have raised concerns regarding the sulfur dioxide (SO2) nitrogen oxides (NOx) and carbon dioxide (CO2) measurements being made under the Acid Rain Program (40 CFR Part 75). In part, these concerns have arisen due to difficulties frequently encountered when conducting linearity tests. Part 75 CEMS operators have also raised concerns about CEMS measurements as they relate to the determination of heat input into the boilers and the subsequent impact on unit heat rate determinations and mass emissions of SO2 (i.e., SO2 allowances). As a result of these concerns and observations made by utilities nationwide, the Electric Power Research Institute (EPRI) has been investigating possible CEMS analyzer biases and linearity effects. This paper summarizes the results of a comprehensive laboratory study conducted by EPRI to investigate possible CEMS measurement errors associated with gaseous analyzer inferences and biases and dilution ratio variability. The primary results of this study were the development of dilution ratio correction algorithms and general guidelines for implementing the algorithms on Part 75 dilution CEMS. Introduction When continuous emissions monitoring system (CEMS) operators began maintaining equipment to comply with Acid Rain Program (40 CFR Part 75), two issues regarding the accuracy of sulfur dioxide (SO2) nitrogen oxides (NOx) and carbon dioxide (CO2) measurements became apparent. First, Part 75 CEMS operators noticed that heat rates calculated using CEMS measurements were consistently higher than heat rate determinations performed using conventional boiler efficiency data. Second, concerns arose due to difficulties frequently encountered when conducting linearity tests. Originally, the heat rate discrepancies were thought to be almost solely a function of the flue gas flow measurements, and the linearity test failures were thought to be caused by a variety of unrelated factors that needed to be addressed on a site-specific basis. To evaluate CEMS flue gas measurement issues, the Electric Power Research Institute (EPRI) funded an investigation of potential flow-related sources of the heat rate discrepancy (Evaluation of Heat Rate Discrepancy from Continuous Emission Monitoring Systems – Final Report TR-108110, July, 1997). Work on the heat rate project clearly showed that there is a flow measurement/flow monitor calibration problem on many of the units. The Environmental Protection Agency’s (EPA) Reference Method 2, which is used to setup (i.e., calibrate) the flow monitors, has a high bias in the presence of angular flow in the stack. During the EPRI heat rate investigation, however, data from several sites indicated that CO2 measurements were also playing a role in the observed discrepancies and that SO2 measurements may be involved, as well. In addition, utilities were still consistently experiencing considerable difficulty with SO2, NOx and CO2 analyzer linearity tests. For many of the data sets that were evaluated during the heat rate project, a significant CO2 high bias between 7 and 12% was observed. Field tests conducted during the heat rate project indicated CO2 analyzer bias in the range of 3 to 3.5%. A smaller, but potentially significant SO2 high bias was also suggested in some cases. To understand better these biases and linearity test difficulties observed during the heat rate project, EPRI has funded the CEMS Analyzer Bias and Linearity Effects (CABLE) laboratory study. The primary objective of the CABLE study was to investigate dilution CEMS analyzer performance and resolve observed differences between dilution CEMS measurements and extractive Reference Method analyzer measurements. In particular, the laboratory study was developed to investigate:
The CABLE laboratory study was designed (building on a previous EPRI dilution probe study) to investigate these issues for both in-stack and out-of-stack dilution probes in a carefully controlled series of tests. At the completion of the laboratory study, algorithms were developed to correct dilution system measurements for changes in dilution ratio. The data was reviewed to identify other errors or biases evident for either type of dilution CEMS and for each of the analyzers. Results of the laboratory study also included the development of general guidelines for implementing the algorithms and discussions regarding several other issues that contributed to gas analyzer measurement errors during the study. Laboratory Test Program To conduct the laboratory study, a series of tests were performed using one "Reference Method" type extractive CEMS, one in-stack dilution CEMS and one out-of-stack dilution CEMS. The tests were conducted at carefully controlled sample conditions for all three CEMS using a specially designed sample chamber. The results of the tests were then compared to establish differences in the CEMS measurements. Differences in the extractive system (considered to be the reference or "Reference Method" for the purposes of this study) and dilution CEMS measurements were then used to evaluate the effects of changes in sample gas conditions. Test Program The CABLE laboratory tests were performed from July 29 through September 4, 1997 during three site visits to the laboratory facility. The first visit was conducted from July 29 through 31, 1997 to set up equipment and to conduct preliminary equipment checks. During the second visit, from August 26 through 29, 1997, an initial series of tests were conducted to collect data at a prescribed set of dilution probe test conditions. During the initial series of tests, a relatively extensive set of experiments were performed to identify and quantify errors in CEMS measurements due to changes in sample gas conditions (i.e., temperature, pressure, and composition), gas constituent interference, analyzer drift and non-linear analyzer response. After completing the initial test series, a brief evaluation of the results was conducted to identify any questionable or unexplainable results. Based on the results of this data review, a third site visit was conducted to collect information under additional dilution probe test conditions. In summary, a total of 78 test runs were performed at a variety of sample chamber conditions:
Prior to beginning the test program, a dilution air quality check, dilution ratio checks on each probe, and analyzer stability checks were performed on each analyzer. A dilution air quality check was performed to insure that analyzer responses were not affected by contaminants in the dilution air (which for the purposes of these experiments was pure nitrogen). Quality checks were performed by zeroing each analyzer with zero air and then checking the response of each analyzer while using dilution air as the zero gas. Dilution ratio checks were performed daily to insure that the initial system setup was consistent from day to day. Ratio checks were performed by calibrating the dilution CEMS CO2 analyzer directly, injecting an 18.00% CO2 gas through the entire system, and then adjusting the dilution air pressure to obtain the desired dilution ratio. Analyzer stability checks were important to insure that test results were not significantly affected by excessive analyzer drift. The stability of each analyzer was verified by conducting zero, low, mid and high calibration error tests for two consecutive days prior to and periodically throughout the test program. Calibration checks on all extractive CEMS analyzers and on each CEMS (dilution and extractive) were performed daily (and in some cases more often) using a zero gas and a Protocol 1 tri-blend calibration gas. Immediately following the daily calibrations, each analyzer was also checked with mid-level and low-level, Protocol 1 tri-blend gases C no adjustment to the analyzers were made based on the mid and low readings. In all cases, the results of the checks and the magnitude of any adjustments were recorded. Throughout the test program, the laboratory temperature and pressure and dilution air pressure were closely monitored and recorded. During each test run, all relevant CEMS sampling chamber and Acublend® parameters were recorded including; sample flow rates, sample cell pressures and vacuums, chamber temperature, ambient temperature and sample gas composition. In addition to these parameters, Scott monitored and recorded sample gas concentrations using a set of extractive analyzers and an extractive FTIR analyzer. Testing was performed such that approximately 10 minutes of stable readings were obtained at each test condition. While, in general, the sampling chamber and CEMS responded quickly to adjustments in pressures and sample gas compositions, varying gas and/or equipment temperatures required some additional equilibration time. During these equilibration periods, the thermocouple readings and CEMS measurements were checked at least once every five minutes until the system had stabilized. This allowed RMB to estimate temperature response times for process and CEMS temperature changes. It should be noted that during these experiments, the probe temperatures seemed to track the changes in chamber temperature without any noticeable or significant delay in CEMS response. During previous dilution probe studies a delayed CEMS response to temperature changes was detected. The lack of any noticeable CEMS temperature equilibration delay during this project may have been caused by the relatively slow change in chamber temperatures – typically, requiring about 20 minutes. As discussed later in this paper, this issue may need to be investigated further during a field study. Test Equipment The equipment used to conduct the laboratory test program included:
The Acublend® system and sampling chamber were designed to allow the generation of all necessary sample gas mixtures (wet and dry) and to control the temperature and pressure of the sample gas being supplied to the tip of each sampling probe. A schematic of the sample chamber is shown in Figure 1. All of the analyzers (with the exception of the extractive CEMS CO2 analyzer) were Thermo Environmental Instruments, Inc. models. The extractive CEMS CO2 analyzer was manufactured by Fuji. All of the analyzers used in the laboratory tests represented a significant portion of the analyzers commonly used by the utility industry for continuous monitoring and by testing contractors for Reference Method testing. The in-stack and out-of-stack probes and the probe control manifolds (which provided dilution air, sample gas and probe temperature control) were manufactured by EPM Environmental. For the purposes of this study, a dilution ratio of 100:1 was used for both dilution probes. The extractive CEMS monitor rack, CO2 analyzer, sample/calibration gas manifold, heated sample line, pumps and pump cabinet, and gas conditioning system (including an electronic condenser) were rented from qualified testing companies. All laboratory testing was conducted at the Scott Specialty Gases facility in Plumsteadville, Pennsylvania. Equipment setup, startup and testing were performed by Scott Specialty Gases’ research technicians and RMB Consulting & Research, Inc. personnel. Summary Of Results When reviewing the laboratory test results, two conclusions became obvious.
In general, the test results indicate that the dilution ratio correction algorithm for in-stack dilution probes will be:
Where: C = Corrected Concentration Cm = Measured Concentration Do = Initial Dilution Ratio Po = Initial Stack Pressure -- during the dilution ratio check P = Stack Pressure MW = Molecular Weight of sample gas MWo = Molecular Weight of Span Gas – used during the dilution ratio check T = Stack Temperature, ° R or ° K To = Initial Stack Temperature, ° R or ° K -- during the dilution ratio check The dilution ratio correction algorithm for out-of-stack dilution probes will be:
Results of the laboratory data analysis indicate that pressure and molecular weight corrections agree well with theoretical formulas; temperature corrections do not. Consequently, as discussed below, the temperature correction component of this algorithm may need to be modified slightly to accommodate site-specific conditions until final solutions can be developed. Furthermore, during this study, the out-of-stack probe was maintained at 450 ° F, well above the sample chamber temperature during all test runs. The in-stack dilution probe was not heated. Depending on the specific probe configuration, ambient conditions and stack gas conditions, the exact temperature correction necessary will change. The issues associated with accurately accounting for temperature fluctuations (i.e., the non-ideal response to temperature changes) appear to be related to fluctuations in the dilution probe ejector pump performance due to changes in dilution air temperature. Note that this problem was observed with both in-stack and out-of-stack dilution probes, but more so with the in-stack probes. In some cases, the dilution air temperature fluctuations may mirror sample gas fluctuations, partially "offsetting" the impact of sample gas temperature shifts; however, in other cases, the dilution air temperature may shift in the opposite direction creating more significant correction problems. Using the equations presented above, utilities should not encounter any temperature adjustment problems except when operating the CEMS at temperatures significantly different from the initial correction "setup" condition (see the discussion below). As previously mentioned, at these larger temperature differences site-specific adjustments to the algorithm may be required. Eventually, an ejector pump performance curve may need to be developed, dilution air temperature may need to be monitored, or some other means of controlling dilution air temperature may need to be developed. Dilution air flow (and consequently, dilution ratio) through the ejector pump is impacted by upstream pump pressure, suction pressure, discharge pressure, the molecular weight of both streams, specific heat capacities and the temperatures of both gas streams (i.e., the sample and dilution air). During the laboratory study, a literature search was conducted to see if theoretical jet pump equations exist. None could be found; pump- specific performance curves will have to be developed if an algorithmic approach is used to fine-tune the dilution probe temperature corrections. During the laboratory tests, there were no noticeable biases or non-linear responses in the extractive or dilution CEMS analyzers. When performing the tests, dilution system CO2 readings were consistently higher than the extractive CEMS CO2 readings -- for any given condition -- by 2 to 5 percent at the mid and low range gas concentrations (11 and 4 %, respectively). When the dilution CEMS CO2 measurements were corrected for changes in molecular weight, however, no CO2 analyzer bias was observed. The CO2 bias observed during the heat rate project field tests (3 to 3.5 %) is consistent with the errors caused by changes in molecular. Note that this error in CO2 measurements will exist for all dilution CEMS not correcting for molecular weight – regardless of whether or not single-blend or tri-blend gases are used. This positive bias will also vary some depending on how the technicians are setting up and operating the analyzers on a daily basis in an attempt to eliminate problems they may have had passing linearity checks. Further investigation of this issue could be performed in the field at plants where and when the bias is observed. One of the more surprising observation during the laboratory study was the significance of desorption and adsorption effects. Even with a relatively short sample line (approximately 30 feet), gas desorption of SO2 when going from high (e.g., 1000 ppm) to low concentrations (e.g., 200 ppm) affected analyzer measurements for 10 minutes or more. Note that based on the extractive CEMS data, the SO2 in the sample chamber appeared to equilibrate quickly. The SO2 adsorption/desorption appeared to be occurring in the dilution sampling system. When measuring stack gas the adsorption and desorption of SO2 probably has no affect on measurements; however, when conducting linearity checks (and varying concentrations significantly over relatively short periods of time) the SO2 results could be affected. Based on dilution ratio checks performed during the test program, dilution ratios varied from day to day due to indistinguishable fluctuations in dilution air pressure. The equipment being used on these dilution air supply systems may not be accurate enough to ensure a constant dilution ratio over extended periods of time. In the future, better ways to control or account for changes in dilution air pressure may need to be developed. EPA is proposing some regulatory modifications (e.g., allowing utilities to use a daily span gas containing 12% CO2) that will reduce the magnitude of the error caused by molecular weight. As molecular weight increases, the volumetric sample flow rate through the critical orifice is reduced, the dilution ratio increases and the gas concentrations measured by the analyzers decrease. Consequently, during daily calibrations with a tri-blend gas containing high CO2 concentrations (16 to 20%), the analyzer readings are adjusted upward to correct for this "decrease" in measured concentrations. Thereafter, at lower CO2 concentrations (observed when sampling and during linearity checks), the volumetric flow rate through the orifice increases, as do the gas concentrations measured by the analyzers, resulting in the positive measurement bias. Based on the result of this test program, by allowing the CEMS to be calibrated at 12% CO2, EPA will not eliminate the variability in dilution ratios caused by changes in molecular weight but will reduce the positive bias to approximately 1 to 3 percent. Interim Solutions When correcting emission measurements for changes in dilution ratio on an ongoing basis, properly implementing an initial CEMS set up process will be paramount. Note that until additional studies can be performed at the site to determine if any site-specific temperature correction adjustments are needed, it is best to perform the setup procedures with the unit on line (i.e., at a typical on-line stack temperature). In general, the procedure begins with a dilution ratio check, which must be performed without any corrections being made to the data from the algorithm presented herein or any other algorithms that may already be installed. A comparison of analyzer outputs and gas concentrations being recorded by the data acquisition and handling system (DAHS) is recommended to verify that no corrections are being performed. While verifying the dilution ratio at a specific set of CEMS operating conditions, important parameters will have to be recorded. These parameters will define an initial CEMS operating condition for which a specific dilution ratio has been determined. After these parameters are entered into the software (in the programmable logic controller (PLC) or the computer) and the correction routine is initiated, all gas concentration measurements will be corrected for any changes in the dilution ratio. The initial setup procedures, to begin making dilution ratio corrections, is presented below.
TO = Target Analog Output, (mA) Co = Calibration Gas Concentration (ppm or %) SF = Scaling Factor (ppm or %)
SF = Scaling Factor (ppm) TD = Target Dilution Ratio
Actual D = Actual Dilution Ratio Ca = Actual CO2 analyzer measurement (ppm) Cd = Concentration of CO2 calibration gas (ppm)
Note that during the initial setup, calibration and linearity operation modes, the correction software will have to assume that %O2 and % H2O equal zero and that orifice pressure is equal to the last stack pressure reading. When sampling stack gas, an assumed moisture and a calculated %O2 (based on the CO2 readings) will have to be used to determine molecular weight. The following equations should be used to calculate molecular weight in the PLC or DAHS software. %O2 = 20.9 - 1.1356(%CO2/(1-%H2O)) %N2 = 100 - (%CO2 + %O2(1-%H2O) + %H2O) MW = (%H2O)(0.18) + (%CO2)(0.44) + (%O2)(1-%H2O)(0.32) + (%N2)(0.28) Where: %O2 = Percent oxygen by volume on a dry basis. When sampling calibration gas (for any reason), %O2 will equal the %O2 concentration in the calibration gas — 0.0 %O2 for a typical dilution CEMS calibration gas. %CO2 = Percent carbon dioxide (on a wet basis when sampling and a dry basis during calibrations) as monitored. %H2O = An assumed value while sampling stack gas, based on recent stack test results, and 0.0 during calibrations. %N2 = Percent nitrogen by volume (on a wet basis when sampling and a dry basis during calibration). MW = Calculated molecular weight of the stack/calibration gas at any point in time. Once the algorithm is in place and operating, if significant temperature swings cause the corrected response to deviate more than about 2 percent, an adjustment may be needed. For in-stack probes, the temperature ratio ranged for the 3rd to the 10th root – overall the 4th root worked best. For out-of-stack probes no temperature correction was typically required; however, in some cases, a very small correction was needed for large temperature changes. Field Study Recommendations The issues and tasks that should be addressed in a field study include:
Acknowledgements The authors acknowledges valued assistance from Dr. Stephen Miller, Dr. Michael Benning and Mr. Jordy Friel of Scott Specialty Gases during the CABLE laboratory study. The authors would like to thank Scott Specialty Gases for fabricating the test chamber and for providing laboratory space, technician assistance, all gases, the use of their Acublend7 dynamic gas blending system and much of the equipment necessary to complete the laboratory study. Special thanks also go to Dr. Dirk Appel of Thermo Environmental Instruments, Inc. and Mr. Hans Brouwers of EPM Environmental for providing analyzers and dilution probe equipment, respectively, for use during the CABLE laboratory study.
Agora Environmental Consulting |